Clinical Research and Its Relation with Improvements In Healthcare
Being a part of Healthcare sciences, Clinical Research determines the safety as well as effectiveness of medicines, diagnostic products, medical devices and treatment plans etc. which are intended for medical treatments of humans. All these may be used to prevent, diagnose or treat the symptoms of diseases. Though clinical practice and clinical research are interrelated, both differ from each other. Diseases or ailments are treated using established treatments in clinical practices whereas clinical research includes the evidence that is collected in order to start treatment.
When a promising molecule or medicine is identified in the lab, then it is subjected to many pre-clinical and animal studies. Through this procedure, the effects and side effects or usage and toxicity of the test medicine is studied. Usually academic medical centers or affiliated research study sites carry out such Clinical research wherein they have access to large number of medical participants. The very ecosystem of Clinical research has grown more complex with the network of sites, numerous Pharma companies and research institutes. This in turn has created the need for new technologies in order to manage data, research and to perform clinical trials.
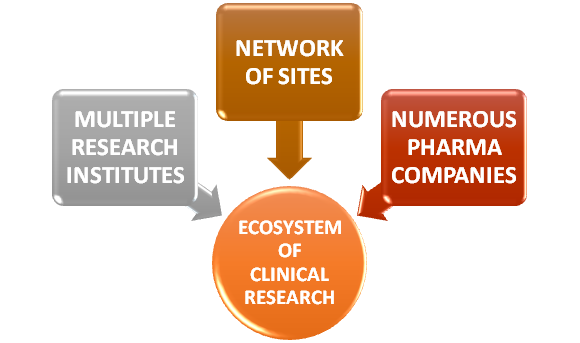
The Governance or Health authorities may need the evidence and authentication in order to use the resources required for research. Even the public understanding about the benefits of such researches is critical.
Required Infrastructure
Infrastructure refers to the health equipment, medical personal and patients, accommodation and so on in which the care is delivered. Clinical research activities demand lot of physical infrastructure like space, specialized equipment and resources to carry out the trials.
Another important aspect of infrastructure is the availability of specialized or experienced human resources in the field of medicine. Resources include physicians, scientists, nurses, laboratory experts, subject domain experts and other health professionals who have experience in the field of clinical research.
Such sophisticated infrastructure demands a huge investment capital and a good resource to manage the whole research unit.
Patient care
The processes involved in taking care of patients make a significant impact on the research outcomes. Such processes of care vary based on research-active and non-research active environments. Research-active institutions are more open for change and they readily receive continuous feedback from the processes. They usually follow the guidelines laid by the clinical practices and deploy new technologies to improve their patient care.
Potential Benefits of Clinical Research
Compared to the positive outcomes of Clinical research, negative clinical trials may be overlooked, even while taking into account of the associated costs and side effects. Such negative impacts are bound to happen and unavoidable while making new inventions to encounter more complex ailments like cancer.
Such clinical trials, often produce fruitful results which can be implemented to eradicate health epidemics or diseases or other ailments. Also, establishing such research units attract outstanding scientists and physicians who have the urge to discover new things in this field.
Phases of Clinical Research
The drugs or medications or therapies which are resulted from Clinical research are subjected to rigorous testing phases which are commonly classified into four phases. Each phase of the approval involves a cycle which is considered as a separate clinical trial and is monitored by highly experienced professionals in this field. These clinical trials may take many years to go through the four phases in order to get an approval by the drug-development authorities. Extensive pre-clinical studies are conducted by the Pharma companies before they roll-out any medications or drugs.
The drugs or medications or therapies passes through the first three phases (say Phase 1, Phase 2 and Phase 3) then the National Regulatory Authority will approve it to be used among the general population. Phase 4 of this process includes the ‘post-approval’ monitoring and studies.
Conclusion
There is a need to analyze or measure the benefits associated with clinical research, which is right now not being done on a large scale. With the high costs associated with the clinical research, it is crucial to perform additional studies to analyze the impact of clinical research and its effectiveness in improving the patient care.
Find a course provider to learn Clinical Research
Java training | J2EE training | J2EE Jboss training | Apache JMeter trainingTake the next step towards your professional goals in Clinical Research
Don't hesitate to talk with our course advisor right now
Receive a call
Contact NowMake a call
+1-732-338-7323Enroll for the next batch
Clinical Research Training Course Program
- Sep 1 2025
- Online
Clinical Research Training Course Program
- Sep 2 2025
- Online
Clinical Research Training Course Program
- Sep 3 2025
- Online
Clinical Research Training Course Program
- Sep 4 2025
- Online
Clinical Research Training Course Program
- Sep 5 2025
- Online
Related blogs on Clinical Research to learn more

CDISC SDTM: Standardizing Clinical Data for Enhanced Research Insights
Learn the importance of CDISC SDTM standards in enhancing research insights by standardizing clinical data.

Exploring the Significance of Medical History in Healthcare
Discover the crucial role of medical history in healthcare, understanding how it informs diagnosis, treatment, and patient care, and its impact on the evolution of medicine.

Key Stages of a Typical Clinical Trial Process Flow
Clinical trials follow a well-defined process flow that is essential for conducting successful and ethical clinical research.

Understanding the Key Stages of a Typical Clinical Trial Process Flow
Discover the critical steps involved in each stage, ensuring a smooth and successful clinical trial process flow.

The Indispensable Role and Importance of Industry Regulations and Standards in Clinical Trials
we have discussed key function in clinical operations, clinical research organization, key function in data management and quality assurance.

Unveiling the Clinical Research Roadmap: A Comprehensive Guide
We have discussed the purpose of Clinical research processes, and what is clinical research process.
Corona Virus (COVID-19), Symptoms, Tests and Safety Measures
Corona Virus (COVID-19), Symptoms, Tests and Safety Measures. Learn the symptoms, tests to undergo, and safety measures to be taken to prevent the virus.

List of Best Clinical Research Training in Atlanta – CRC, CRA, MSCR Studies
We have collected a list of reputed institutes to those interested in pursuing a career in clinical and/or translational research. Pick from the list to have your dream come true career in medicine.
Healthcare Sector increasing the focus in Big Data and Technology
Here the role of technology business mergers and big data firms within the healthcare frontiers comes into play. The business mergers of technology, as well as the big data firms in the healthcare sector, highlights a trend viral within the clinical

7 Ethics for Clinical Researchers
Clinical trials conducted by the researchers involve a human volunteer who is willing to be subject to adhere new findings or a medical experiment. For that, a proper consent is acquired from the volunteer before conducting the trials.
Latest blogs on technology to explore

Understanding Artificial Intelligence: Hype, Reality, and the Road Ahead
Explore the reality of Artificial Intelligence (AI) — its impact, how it works, and its potential risks. Understand AI's benefits, challenges, and how to navigate its role in shaping industries and everyday life with expert training programs

How Much Do Healthcare Administrators Make?
Discover how much healthcare administrators make, the importance of healthcare, career opportunities, and potential job roles. Learn about salary ranges, career growth, and training programs with Sulekha to kickstart your healthcare administration jo

How to Gain the High-Income Skills Employers Are Looking For?
Discover top high-income skills like software development, data analysis, AI, and project management that employers seek. Learn key skills and growth opportunities to boost your career.

What Companies Expect from Product Managers in 2025: Skills, Tools, and Trends
Explore what companies expect from Product Managers in 2025, including essential skills, tools, certifications, and salary trends. Learn how to stay ahead in a rapidly evolving, tech-driven product management landscape.

Breaking Into AI Engineering: Skills, Salaries, and Demand in the US
Discover how to break into AI engineering with insights on essential skills, salary expectations, and rising demand in the US. Learn about career paths, certifications, and how to succeed in one of tech’s fastest-growing fields.

Cybersecurity Training: Powering Digital Defense
Explore top cybersecurity training programs in the USA to meet rising demand in digital defense. Learn about certifications, salaries, and career opportunities in this high-growth field.

Why Pursue Data Science Training?
Empower your career in a data-driven world. Learn why data science training is crucial for high-demand jobs, informed decisions, and staying ahead with essential skills.

What Does a Cybersecurity Analyst Do? 2025
Discover the vital role of a Cybersecurity Analyst in 2025, protecting organizations from evolving cyber threats through monitoring, threat assessment, and incident response. Learn about career paths, key skills, certifications, and why now is the be

Artificial intelligence in healthcare: Medical and Diagnosis field
Artificial intelligence in healthcare: Medical and Diagnosis field

iOS 18.5 Is Here: 7 Reasons You Should Update Right Now
In this blog, we shall discuss Apple releases iOS 18.5 with new features and bug fixes