Clinical Research in Sollers College
We provide professional development programs in the field of Clinical Research and assist our graduates in securing jobs and externships as they reposition themselves in the market.
Collaborative Learning and Career Building
At the end of most Clinical Research lessons, you'll have access to an online discussion. Engaging actively and constructively in these discussions can significantly boost your Clinical Research career development. By offering help or seeking assistance from the trainers, you’ll build meaningful relationships and create valuable professional connections.
These discussions are more than just a conversation to share ideas—they're designed to accelerate your Clinical Research learning journey. That's why it is made an essential part of our courses: to support your growth and help you enhance your Clinical Research skills through collaboration and shared insights.
Advantages of enrolling up for Clinical Research

- Online Training
- Classroom Training
- Placements
Details to know about Clinical Research

Clinical Research PROGRAM AT SOLLERS
What programs are we offering in Clinical Research?
We are offering 2 programs in Clinical Research:
- Clinical Trial Management
- Advanced Clinical Research
a) Clinical Trial Management (300 hours) with 20 weeks internship
Tuition of Advanced Drug Safety and Pharmacovigilance is USD 7500
Tuition:
The tuition of Clinical Trial Management program is USD 7500
Objective of the program
The Clinical Trial Management is a highly-selective program for students with a strong background in any Biological-Computer-Mathematics/Statistical-or Regulatory Science area that includes Medicine, Nursing, Dentistry, Pharmacy, Microbiology, Biotechnology, Biochemistry, similar areas. Students with a background in Information Technology, Biostatistics, and other general Commerce and Finance background can also take this as an additional specialty option after some prerequisite training.
This course provides training for students to learn how the Clinical Trial Management process works. Students will learn about the development of complete regulatory activities involved in Creating Documents; and in Planning, Organizing, Monitoring, Recording, Analysis and Reporting of Clinical Trials in Human Beings. The program provides additional opportunities to learn about recent advancements as well as specializations in e-Clinical Technology.
Modules covered in the training
Study Start Up
Module 1
- Phases of the Clinical Trial
- ICH-GCP Guidelines
- Study Feasibility/Site Selection
Module 2
- IRB/IEC
- Budgets
- Contracts
Module 3
- Essential Documents (Prior to Study Start)
- Financial Disclosure Form
- Clinical Trial Protocol
Module 4
- Investigator Brochure
- Informed Consent Process
- Monitoring- General introduction to all thetypes of visits
Study Conduct
Module 5
- Site Monitoring Visits (Include Remote/Central Risk-Based Monitoring Concept)
- Randomization/Blinding
- Recruitment/Retention/Compliance
Module 6
- Adverse Events/Serious Adverse Events
- Drug Advertisement
- HIPAA Regulations
Module 7
- Medical Devices
- Fraud/Misconduct/Potential Liability
Module 8
- FDA Inspection
- Essential Documents (During Study Conduct)
Study Closeout
Module 9
- Site Close-Out Visit (On-Site/Remote activities)
- Statistical Analysis
Module 10
- Investigational Drug Application
- New Drug Application
- eCTD (Common Technical Document)
Module 11
- Essential Documents (After Study Close-Out)
- Risk-Based Monitoring
Module 12
- Evidence Based Medicine
- Personalized Medicine
- Big Data
20 weeks internship with hands-on training on:
- CTMS - Clinical Trial Management system
- EDC-Electronic Data Capture Management system
- eTMF-Electronic Trial Master File
- Case studies and Real Time Scenarios taught by industry experts
b) Advanced Clinical Research (150 hours)
Tuition:
The tuition of Advanced Clinical Research program is USD 4000
Objective of the program
The Advanced Clinical Research provides a thorough understanding of Clinical Research process including training on GCP/ICH guidelines.
Modules covered in the training
Study Start Up
Module 1
- Phases of the Clinical Trial
- ICH-GCP Guidelines
- Study Feasibility/Site Selection
Module 2
- IRB/IEC
- Budgets
- Contracts
Module 3
- Essential Documents (Prior to Study Start)
- Financial Disclosure Form
- Clinical Trial Protocol
Module 4
- Investigator Brochure
- Informed Consent Process
- Monitoring- General introduction to all thetypes of visits
Study Conduct
Module 5
- Site Monitoring Visits (Include Remote/Central Risk-Based Monitoring Concept)
- Randomization/Blinding
- Recruitment/Retention/Compliance
Module 6
- Adverse Events/Serious Adverse Events
- Drug Advertisement
- HIPAA Regulations
Module 7
- Medical Devices
- Fraud/Misconduct/Potential Liability
Module 8
- FDA Inspection
- Essential Documents (During Study Conduct)
Study Closeout
Module 9
- Site Close-Out Visit (On-Site/Remote activities)
- Statistical Analysis
Module 10
- Investigational Drug Application
- New Drug Application
- eCTD (Common Technical Document)
Module 11
- Essential Documents (After Study Close-Out)
- Risk-Based Monitoring
Module 12
- Evidence Based Medicine
- Personalized Medicine
- Big Data
2) What is Sollers USP(Unique Selling Proposition)?
- Hands on training on industry relevant tools like CTMS, eDC, ETMF
- Faculty includes highly experienced industry practitioners
- Certified course material provided for training
- Internship opportunities are available
- Our career services team assist with placements
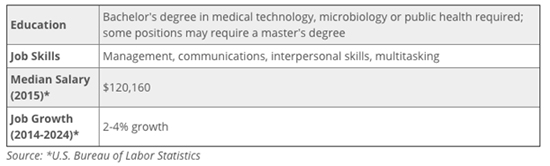
3) What are the job and career prospects for a Pharmacovigilance/ Drug Safety professional?
A competitive income and a strong outlook for clinical research associates make working as a CRA a great career choice. According to Indeed.com, the average salary for CRAs as of August 31, 2015, is $95,000, but some earn over $125,000.
Growth is projected to be stronger than the average job, at 36.4% in the ten year period from 2012-2022, according to the Bureau of Labor Statistics.
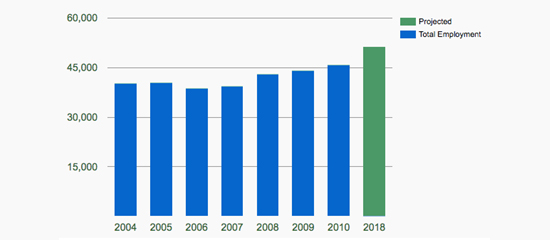
Above figure depicts the demand for Clinical Research Coordinators with an expected 5590 new jobs filled by 2018
Career track in Drug Safety and Pharmacovigilance:
- Clinical Research Coordinator
- Clinical Research Associate(I/II)
- Clinical Document Specialist
- Clinical Research Scientist
- Clinical Operations Manager
- Clinical Director
Who hires Clinical Research professionals?
Professionals trained in Clinical Research have lucrative career options with balanced life style in:
- Pharmaceutical/ Biotechnology Companies
- Contract Research Organizations (CROs)
- Knowledge Process Outsourcing (KPOs)/ Consulting organizations such as Accenture, Cognizant, etc. and Regulatory Authorities such as DCG(I), FDA & CDSCO.
Where does Sollers students find placement?
- Our students have got into positions with the pharmaceutical companies and CROs like Merck, Sanofi, Pfizer, BMS, J&J, PRA Health Sciences, Covance, Celgene, Paraxel etc to name a few.
FAQs Clinical Research from Sollers College
Where is the course conducted?
The Clinical Research is conducted from Edison to attend in classroom and in online, accessible via a dedicated platform from anywhere.
What are the available learning modes for Clinical Research?
Sollers College offers online, in-person, and hybrid learning options
What facilities are provided for students enrolled in this course?
Sollers College offers access to online resources, expert lectures, practical labs, student support community, 100% Job assistance and Guaranteed classes.
Who will be conducting the training for this course?
The training for Clinical Research will be conducted by experienced industry professionals, subject matter experts and certified instructors.
What topics are covered in the course curriculum?
The course covers topics according to the skills and understanding you have on the subject.
| Date & time | Module | Training title | Mode | Training provider / fee | Register |
| Clinical Research | Clinical Research Provided By Sollers College | Clinical Research institutes |
Online | |||
| Clinical Research | Clinical Research Provided By Sollers College | Clinical Research institutes |
Online | |||
| Clinical Research | Clinical Research Provided By Sollers College | Clinical Research institutes |
Online |
 +1 –732-338-7323
+1 –732-338-7323


